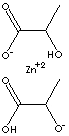| ZINC LACTATE DIHYDRATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 16039-53-5 |
|
| EINECS NO. | 240-178-9 | |
| FORMULA |
Zn(C3H5O3)2·2H2O | |
| MOL WT. | 279.51 | |
|
H.S. CODE |
2918.11 | |
| TOXICITY |
| |
| SYNONYMS | Lactic Acid, Zinc Salt; | |
| Zinkdilactat (German); Dlactato de cinc (Spanish); Dilactate de zinc (French); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White crystals | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
|
REFRACTIVE INDEX |
| |
|
NFPA RATINGS |
||
|
AUTOIGNITION |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
Lactic acid (chemically, alpha or 2-Hydroxypropionic acid) takes roles in metabolic processes in the body; in red blood and in
skeletal muscle tissues as a product of glucose and glycogen metabolism. Lactic acid is an "alpha
hydroxy acid: which has a hydroxyl group on the
carbon atom next to the acid group. If the hydroxy group is on the second carbon
next to the acid group, it is called beta-hydroxy acid. Lactic acid is converted
in vivo to pyruvic acid (an alpha keto acid) which occurs as an intermediate
product in carbohydrate and protein metabolism in the body. Lactic
acid occurs as two optical isomers since the central carbon atom is bound to
four different groups; a dextro and a levo form ( or an inactive racemic mixture
of the two); only the levo form takes part in animal metabolism. Lactic acid is
present in sour milk and dairy products such as cheese, yogurt, and koumiss,
leban, wines. Lactic acid causes tooth decay since lactic acid bacteria
operates in the mouth. Although it can be prepared by chemical synthesis,
production of lactic acid by fermentation of glucose and other sugar substances
in the presence of alkaline such as lime or calcium carbonate is a less
expensive method. The six-carbon glucose molecule is broken down to two
molecules of the three-carbon compounds (lactic acid), during this anaerobic
condition. Synthetic lactic acid is used commercially in tanning leather and
dyeing wool; as a flavouring agent and preservative in food processing and
carbonated beverages; and as a raw material in making plastics, solvents, inks,
and lacquers; as a catalyst in numerous chemical processes. Lactic Acid is
available as aqueous solutions of various concentrations, usually 22 - 85
percent (pure lactic acid is a colourless, crystalline substance.) Some examples
of lactates (salts or esters of lactic acid) are:
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White crystals | |
|
ASSAY (Zn) |
22.0% min | |
|
IRON |
5ppm max | |
| SULFATE |
50ppm max | |
| CHLORIDE |
20ppm max | |
|
LEAD |
5ppm max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in Bag, 20mts in Container | |
| HAZARD CLASS | ||
| UN NO. | ||
| GENERAL DESCRIPTION OF ZINC MINERAL | ||
| Zinc is an essential mineral having a role in the maintenance of the body's nervous and immune systems (T-cell function). This mineral is involved in the biochemical reactions as an antioxidant in the healing process and develops normal tissues Zinc is a cofactor in enzymatic reactions such as protein synthesis polymerases and in carbonic acid anhydrase. Zinc maintains the body's alkaline balance. Zinc finger, a structural domain found in many gene-regulatory proteins, is a component of hydrophobic hormones acting stabilizing the biomembrane structures and cell membrane metabolism. Zinc deficiencies may result in prolonged wound healing, delayed sexual maturation, mental lethargy, skin changes, and susceptibility to infections. Gluconate and citrate forms are mainly used as zinc supplements. They are easily absorbed by the body. | ||